1. Fungi--probably pythium fungi, cause plant tissues to deteriorate consequently converting duckweed into a meal for micro-organisms (Suren, 1989). Pythium blight starts small and spreads until it eventually infects all the duckweed. (Rejmankova, 1986; website http://www.mobot.org/jwcross/duckweed/duckweed-pests.html)
Methods to prevent and/or eliminate fungi:
a) Fungicide. Apply fungicide like Ridomil Gold EC at a rate of 0.3uL/L-nutrient solution. To do this, you can make up "Subdue" solution (0.3ml-Ridomil GoldEC/L) and then apply "Subdue" solution at a rate of 1mL-"Subdue"/L-nutrient solution. I only had to apply this fungicide to my 100L reactor once to get rid of all the fungi. I had no problem with the fungi until I began to experiment with lower duckweed densities (below 30g(dry)/m^2) and low nutrient solutions (<1ppm P and N). After the fungi reappeared following the latter growth conditions, I accidentally applied Ridomil GoldEC at a rate of 1mL/L-soln. Oops! I came back the next day to find a room that smelled like paint thinner and a reactor void of duckweed (all of it sunk to the bottom of the reactor).
b) Silicon. Add silicon to nutrient solution which might have the effect of "toughening" cell tissue to resist fungi and disease. The USU Crop Physiology Laboratory has noticed that their hydroponic solutions containing silicon are more resistant to disease. They have a recipe to produce 2KOH + SiO2 --> K2SiO3 + H2O. The instructions to make this are as follows: First, dissolved 44.9g KOH in ~3.5L distilled water; Second, stir until clear (~15 min.); Third, add 24 g SiO2 (fumed silica--before to use a fume hood and/or filter mask); Fourth, stir until clear (~4-8 hrs.); and Fifth, bring volume to 4 L. Note: they have also noticed disease resistance by simply adding chunks of potassium silicate glass to the sediment to supply the silicon.
 Figure 2: Duckweed fronds 3-7 days after Fig. 1.
Figure 2: Duckweed fronds 3-7 days after Fig. 1. Figure 3: Duckweed fronds completely infected by fungi and being decomposed by micro-organisms.
Figure 3: Duckweed fronds completely infected by fungi and being decomposed by micro-organisms.c) Temperature. Regarding fungi with duckweed and temperature effect, Elias Landolt wrote:
8. Fungi. The hypochytridiomycetes Reessia amoeboides and Reessia lemnae live endobiotically in dying Lemnacea, accoding to Wagner (1969) and Kandeler (1979). Colbaugh (1981) reports of a lethal foliar blight of Lemnaceae in water cultures, which is caused by the oomycete Pythium aphanidermatus. The reduction occurs due to foliar blight and dying of the fronds. Greatest foliar blighting activity occurs at temperatures of 24'C and 27'C (better than at 18'C, 21'C, and 30'C). Rejmankova et al. (1986) isolated Pythium myriophyllum from L. gibba growing in a dairy farm of Louisiana. The authors were able to show that this gungus is the cause of duckweed kills. Under natural conditions and temperatures above 22'C the amount of duckweeds killed by the gungus grows exponentially and the whole stand dies within several days. Six species of lemnaceae have been tested in the laboratory: L. gibba, L. minor, and S. polyrrhiza proved to be most susceptible to the fungla infection. L. valdiviana showed more resistance whereas L. aequinoctialis and S. punctata never exhibited symptoms of desease. Optimum temperature for infection was about 32'C. It is interesting to note that the susceptibility to a fungal disease might be a factor limiting the distribution of certain Lemnaceae species. Rhizoctonia solani is able to infect L. minor, but the plants only get small irregular lesions (Joyner and Freeman 1973). A smut, tracya lemnae, is known from Spirodela (Fisher 1953, Zogg 1985).2. Algae--when duckweed densities are low, then algae grow by the light that otherwise would be absorbed by floating duckweed. Algae grows in the water column and the surface. It can attach to duckweed tissue. I've personally observed that air bubble form underneath duckweed in solution with algae--cutting off its interface directly with the water column. Elias Landolt said:
Citer from (Landolt, 1986, pp.194-195)
Algae are most competitive with Lemnaceae in nutrient-rich waters. Filiform algae, which form dense mats on the surface of the water (e.g. Spirogyra) especially can prevent Lemnaceae from spreading successfully. Very often, the algae cover is raised by development of gas, thus breaking the contact of the Lemnaceae with the water and causing the drying of fronds.Further reading: (Szabo, 1998/2003/2005; Roijackers, 2004; Smart, 1985)
Cited from (Landolt, 1986, p.203)
Ways to reduce/eliminate the growth of algae with duckweed include:
a) rinse duckweed in 0.05% sodium hypochlorite soln. (aka. bleach)--this is comparable to chemotherapy for duckweed plants and it's a matter of "survival of the fittest." You only need to rinse for 5-30 seconds. I tried this procedure once and rinsed fronds for 30 seconds which destroyed virtually all of the fronds (and algae).
b) as part of "a" growing cultures in the lab should be as aseptic as possible (i.e. autoclave or filter nutrient solution, laminar flow hood, autoclaved glassware, etc.). This is difficult for me to do which is probably why I seem to always end up with algae showing up.
c) foil or dark material to cover all but the surface of the growing vessel reducing the light available for algae growth beneath the water suface.
d) maintain a crop density of at least 20-30g(dry)/m^2; anything lower than this allows too much light to pass through the duckweed cover.
e) filter using sand and/or diatomaceous earth (Naghavi, 1986)
f) periodic spraying ponds with algicide copper sulphate at a concentration of 2mg/L; spray at noon when temperatures and algae concentrations are high. This algicide procedure was used to remove filamentous alga Oedognium (Edwards, 1992).
 Figure 6: Signs of algae infestation (progressively worse L to R).
Figure 6: Signs of algae infestation (progressively worse L to R). Figure 7 (top-bottom pairs): Logan City wastewater w/o and w/ chlorine;
Figure 7 (top-bottom pairs): Logan City wastewater w/o and w/ chlorine;Wellsville City wastewater; Hydrosol nutrient solution.
 Figure 8: Bioassays (APHA Std. Method 8211) by frond count. Logan w/ and w/o chlorine.
Figure 8: Bioassays (APHA Std. Method 8211) by frond count. Logan w/ and w/o chlorine. Figure 9: Bioassay method 8211 Hydrosol and Wellsville City ww solutions.
Figure 9: Bioassay method 8211 Hydrosol and Wellsville City ww solutions.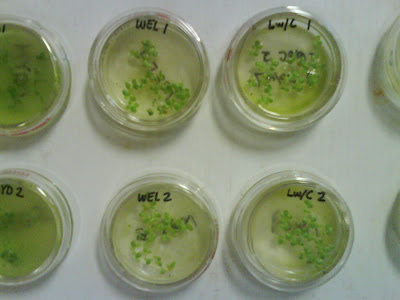 Figure 10: Bioassay method 8211; 3-5 days (typ. 96 hrs.); Wellville City and Logan City w/Cl ww.
Figure 10: Bioassay method 8211; 3-5 days (typ. 96 hrs.); Wellville City and Logan City w/Cl ww. Figure 11: Duckweed plants following bioassay;
Figure 11: Duckweed plants following bioassay;removed one-by-one for frond/colony counting.
 Figure 12: More duckweed plants following bioassay;
Figure 12: More duckweed plants following bioassay;removed one-by-one for frond/colony counting.
 Figure 13: Bioassay results comparing effect of nutrient solutions and chlorination on duckweed growth. It appears that nutrient solution has a larger effect on duckweed growth than whether or not it has been chlorinated (see below).
Figure 13: Bioassay results comparing effect of nutrient solutions and chlorination on duckweed growth. It appears that nutrient solution has a larger effect on duckweed growth than whether or not it has been chlorinated (see below).
Figure 14: Boxplots showing effect of nutrient solution and chlorination on duckweed growth. Results show that duckweed growth slows over time (i.e. harvest more frequently) and chlorination effect is negligible.
References:
Suren, A.M., "Histological changes in macrophyte tissue during decomposition," Aquatic Botany, 33 (1989) 27-40.
Rejmankova, E., "Dynamics of fungal infection in duckweeds (Lemnacea)", Veroff.Geobot.Inst.ETH, Zurich, 87 (1986), pp 178-189.
Landolt, E. (1986): The family of lemnaceae-a monographic study. Vol. 1 of the monograph: Morphology; karyology; ecology;geographic distribution; systematic position; nomenclature; descriptions. Published in the "Veroffentilichungen des Geobotanischen Institutes ETC, Stiftung Rubel, Zurich." This is also listed as vol. 2 (No. 71) of publications on "Biosystematic investigations in the family of duckweeds Lemnaceae."
Szabo, S., et al., "Influences of nine algal species isolated from duckweed-covered sewage miniponds on Lemna gibba L.," Aquatic Botany 60 (1998) pp189-195.
Szabo, S., et al., "A simple method for analysing the effects of algae on the growth of Lemna and preventing algal growth in duckweed bioassays," Arch. Hydrobiol. 157 (4) pp.567-575 (2003).
Szabo, S., et al., "The strength of limiting factors for duckweed during algal competition," Arch. Hydrobiol. 164 (1) pp. 127-140 (2005).
Roijackers, R., et al., "Experimental analysis of the competition between algae and duckweed," Arch. Hydrobiol. 160 (3) pp. 401-412 (2004).
Smart, R.M., et al., "Laboratory culture of submersed freshwater macrophytes on natural sediments," Aquatic Botany, 21 (1985) pp251-263.
Naghavi, B., et al., "Algae removal by fine sand/silt filtration," Wat. Res. (1986) Vol 20, No. 3, pp. 377-383.
Edwards, P., et al., "Cultivation of Duckweeds in septage-loaded earthen ponds," Bioresource Technology, 40, pp.109-117 (1992).
Watkins, C., "The toxicity of chlorine to a common vascular aquatic plant," Water Res. 8 (1984) pp. 1037-1043.
Culley, D.D., et al., "Production, chemical quality, and use of duckweed (Lemnaceae) in aquaculture, waste management, and animal feeds," Journal of the World Mariculture Society, Vol. 12 (2), pp.27-49 (1981).



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.