Click Here to View PaperTitle: Investigating Duckweed for Phytoremediation and as a
Toxicity Indicator of Chemicals
Created: May 2010
Author: Jon Farrell
Project: USU Environmental Toxicology 6270
Abstract:
Duckweed is an aquatic plant that can hyperaccumulate various nutrients and toxic metals, and can metabolize several organic chemicals. Duckweed is uniquely suited for phytoremediation of polluted surface water and for toxicity testing. Polyaromatic hydrocarbons (PAHs) are particularly toxic to duckweed and other plants due to metabolites, formed by UV photo-degradation of the parent compound, which ultimately lead to lower photosynthetic rates. On the other hand, plants like duckweed are useful from an environmental standpoint because they contribute to 45% of PAH degradation. This report reviews several studies that investigated the ability of plants like duckweed to uptake specific chemicals of concern. It also investigated the toxic effect certain chemicals like PAHs, As, Se, Cd, Cu, and chlorophenols had on duckweed—which in some instances like Cd, showed how toxic chemicals bioaccumulate through the food chain to humans. These toxic chemicals caused phytotoxicity via CYP-450 oxidation, free radicals, DNA damage, and cell membrane damage.
Background:
Duckweed has been researched extensively for more than 40 years (Clatworthy et al., 1960; Culley et al., 1981) as an ecological way of remediating polluted water and as a toxicity indicator. Researchers have investigated its ability to remove metals, inorganic nutrients, and more recently, organic chemicals including pharmaceuticals. Regarding phytoremediation, it has been noted that the best plants, including duckweed, hyperaccumulate the chemical(s) of concern, grow quickly, and harvest easily. Duckweed hyperaccumulates several toxic metals including Cd, Cu, and Se (Lone et al., 2008); has a high relative growth rate (RGR) of 0.06 up to 0.31 (Chaipraprat et al., 2005); and is a native species throughout the United States and the world (see Fig. 1), and simplifies harvesting due to the fact that it floats on water. These same characteristics make it ideally suited for toxicity testing because it uptakes many chemicals, grows on water including wastewater, and is adaptable to many environments. Both the American Public Health Association (APHA) and Environment Canada have official testing methods for duckweed toxicity testing.

Retrieved from
USDA Plants Database Figure 1: Distribution of Lemna minor (a.k.a. duckweed)
The majority of research concentrates on using duckweed for phytoremediation. A handful of studies have concentrated on using duckweed merely as toxicity indicators of aquatic environments (Tront et al., 2007; Saygideger et al., 2004) and a few of these studies have used duckweed to identify sentinel species. Sentinel species identify hazards to human health, similar to a canary in a coal mine. In a study done in Louisiana, Cd-enriched duckweed was fed to crayfish and the effect of bioaccumulation was seen as acetylcholinesterase activity diminished (Devi et al., 1996). The crayfish were labeled as sentinel species since they are only one step in the food chain away from humans. Duckweed not only shows promise for remediating polluted water, it also has proved itself in identifying harmful chemicals—some of which may end up on a dinner plate. In a very recent report from the President’s Cancer Panel, it was pointed out that 41% of Americans will be diagnosed with cancer and 21% will die from it. The United States was criticized of having a “reactionary rather than precautionary” regulatory approach. And the report pointed out that “only a few hundred of the more than 80,000 chemicals in use in the United States have been tested for safety” (USDHHS). While duckweed does not always uptake the same chemicals as humans nor experience the same toxic effects, at times it does behave similarly and also provides a relatively quick indication of hazards from certain chemicals like pharmaceuticals (Brain, 2004) and Polyaromatic Hydrocarbons (Kummerova et al., 2007).
While duckweed has been employed in full-scale remediation projects (Alaerts et al., 1996; Donahue, 2009), the majority of research has been conducted in laboratory settings and shows the effect of specific chemicals on the plant rather than entire ecological system (Bonairdi, Linton et al., 1998). Some compelling full-scale projects might include studies in North Carolina investigating duckweed as a means of cleaning up swine wastewater, specifically to remove nitrogen and phosphorus that would otherwise cause eutrophication and river impairment downstream from where lagoons discharge into rivers (Chaipraprat et al., 2005). In additional to removing inorganic nutrients, duckweed has also been shown to remove uranium and arsenic from mine drainage in Saxon, Germany (Makandawire 1 & 2) and hard to remove selenium (Ornes et al., 1991). There are several physiological characteristics that make duckweed suitable to remediation and toxicity testing.
Plant Physiology:
Duckweed is the common name for the Lemnaceae family of plants, with species like Lemna minor, Lemna Gibba, and Spirodela Polyrhizza. Duckweed is one of the smallest macrophytes, a monocot, angiosperm, and C-3 plant—which allows it to grow in colder climates with only 5-7 month growing seasons. Since water freezes in the winter and duckweed grows on water, it does best in warmer climates. Duckweed can produce seeds called turions, but typically reproduces asexually by growing more daughter fronds which eventually separate into their own colonies of 2-4 fronds.
Phytoremediation began as an interest to understand how nutrient metals were physiologically taken up by plants. These early studies looked into what factors made nutrients available to the plant from the soil. Phytoremediation as it’s known today, is concerned with using plants to remove harmful chemicals from the environment. Phytoremediation typically involves large fast growing plants rooted in soil that hyperaccumulate chemicals of concern. These plants include willows and poplars (Yifru et al., 2006); however, duckweed and other floating plants are uniquely able to remediate large water bodies without soil. Abiotic factors like pH and redox potential are the main drivers of metal availability in plants, but uptake mechanism are not solely limited to pH and ORP. Plant uptake of solutes and water depend on mechanisms such as osmotic potential, vapor potential, enzymes, microbes, and the physical nature of the chemical and the plant.
Osmotic potential is important to understanding plant-chemical relationships. Osmotic potential is the pressure gradient between the soil and plant, and is governed by the equation:

Where T=Total osmotic potential (approx. -2 to -1 Mpa); S=Solute potential (directly proportional to concentration and ionization of salts); P=Turdor potential (positive pressure as plant cells fill with water); M=Matrix potential (affinity of soils for water); and Z=pressure due to gravity (negligible)
Soil and healthy plants have a negative pressure. Depending on which pressure is greater drives where the water will go. Soil pressure is driven primarily by S + M while plant pressure is driven by S + P. If the osmotic pressure is greater in the substrate (i.e. soil or water) due to concentrated salts then the plant will not be successful at taking up chemicals.
Vapor pressure is essential to plant physiology and phytoremediation because it decreases P. as water transpires from the plant, thus drawing more water into the plant through the roots. Vapor pressure also governs whether certain chemicals, like trichloroethylene will volatilize through plant leaves (Orchard et al., 2000).
pH and redox potential effect the protonation of chemical compounds which in turn determines whether it is in a bioavailable form (Tront et al., 2007). pH and redox can be influenced by microbes near plant surfaces that hydrolyze chemicals (Makandawire). Enzymes enable certain chemicals (i.e. PO4-P) to be actively transported into and utilized by the plant functional groups.
The physical characteristics of chemicals and plants determine the type of chemicals taken up by plants and kinetic rate at which they’re taken up. Physical characteristics of the chemical include the octanol/water coefficient (Kow), molecular charge, and size. Typically, plants uptake organic chemicals with moderate to low hydrophobic Kow’s ranging from 0.5-3.5 into their tissue (i.e. lipid compounds); while on the other hand, aqueous chemicals (i.e. Kow<0.0) are easily transferred through plants via the xylem (Kim et al., 2004) or in some cases not taken up at all if the pKa is too low (Boutonnet). The uptake of hydrophobic to hydrophilic compounds by the plant via different mechanisms is shown via the root’s anatomy.
The plant’s roots can be visualized as circular layers of cells, beginning from the outermost layer: epidermis, cortex, endodermis, pericycle, and xylem. Different transport mechanisms exist to transfer chemicals through the different layers. According to Taiz, “Mineral nutrients absorbed by the root are carried to the shoot by the transpiration stream moving through the xylem” (Taiz et al., 1998) via the apoplast. Hydrophobic compounds don’t move through the apoplast; rather, they move through a network of interconnected lipid-cells known as the symplast (Taiz et al., 1998). According to Kim, non-aqueous chemicals (i.e. Kow>0) require symplastic movement through inner cells, aqueous chemicals require apoplastic movement through cell walls, and inorganic nutrients require “specific carrier- and channel-proteins” (Kim et al, 2004). Once a chemical is taken up by a plant it can be stored, metabolized (i.e. assimiliated), mineralized, or volatilized.
Physiological characteristics of plants (i.e. floating vs. rooted) make specific species more adept at removing specific chemicals. Individual laboratory tests frequently concentrate on one specie’s ability to uptake one type of chemical. In reality, ecological systems can contain multiple chemicals of concern. Some studies have looked into using multiple types of plants, each one with a particular ability to remove a specific chemical (Ornes et al. 1991). Other studies have observed competition between species that promote or inhibit multiple species (Clatworthy et al., 1960; Edwards et al., 1992, Wang et al., 2002).
Materials and Methods/Indicators of Phytotoxicity:
The majority of these studies investigating the use of duckweed and other plants for chemical uptake and toxicity were performed in the laboratory. One advantage of lab studies is that they “generate information about the fate of organic chemicals prior to field-scale tests because laboratory tests are less expensive, easier to control, and better enable investigators to elucidate fate mechanisms” (Kim et al., 2004). Each study regarding duckweed has a slightly different focus, ranging from finding uptake rates to discovering mechanisms of action. Due to a variety of focuses, each study also performed different tests (i.e. substrate solutions) and analyzed the results differently (i.e. chemical concentration vs. EC50). To begin with, a decision must be made as to what solution should be used to grow the plants, and whether or not the plants will be grown in large-several liter reactors, small shake flasks, or petri dishes. Standard methods exist for carrying out toxicity tests, but even these vary depending on what chemicals are being tested. For example, one test might require chelating agents in the nutrient solutions that in turn might make certain chemicals unavailable to the plant (Saygidegar). Nutrient solutions range from Hoagland, to Huntner, to industrial flue gas water (Sundberg et al., 2006), to wastewater.
The physical environment is often measured for light intensity, temperature, pH, ORP, and DO (Nzengung et al., 2004). These variables were used by Nzengung to demonstrate that uptake of perchlorate increased in aerobic conditions and enriched nitrate conditions; however, rhizodegradation decreased-and rhizodegradation is often preferred over plant uptake since it destroys perchlorate. In another study, pH measurements and comparison between pKa and percent protonated species revealed that chemical uptake utilized both abiotic (i.e. pH) and biotic (enzymatic transformation) mechanisms (Tront et al., 2007).
Enzymatic activity within plants were measured frequently to indicate toxicity. Activation of antioxidative enzymes were associated with free radical formation and subsequent physiological damage (Wang et al., 2004). Whereas, deactivation of acetylcholinesterase enzymes were associated with phytotoxicity due to Cd hyperaccumulation (Devi et al., 1996).
Mass balances showed the fate and motility of chemicals. The majority of studies reported the Bio Concentration Factor (BCF) which is the ratio of the amount of chemical in the plant tissue compared to the amount in the substrate solution. High BCF (typ. >1000) represent hyperaccumulation (Odjegba et al., 2004). A number of studies used C14 labeled chemicals to show the ratio of chemical in roots, to shoots, to the unaccounted chemical volatilized or degraded (Botcher).
Various extraction solutions provided similar data as C14 labeled compounds and were used to identify the chemicals connected to the roots and those in the shoots (Vadas et al., 2007), identified by the Root Concentration Factor (RCF). Similarly, extraction can be used to determine the amount of chemical adsorbed vs. absorbed/assimilated in plants. Comparable to BCF, the Transpiration Stream Concentration Factor (TSCF) shows the ratio of chemical in the transpiration stream to that in the external solute (Gomez-Hermosillo et al., 2006).
A study by Utah State University (USU) recognized the importance of carefully measuring and controlling the air to determine the fraction of chemical that was volatilized by plants. Trichloroethylene can be volatilized by fruit trees and poplar trees. USU created growth chambers specifically designed to keep track of CO2in/out, O2in/out, and also utilized CO2 traps to keep track of the fraction of mineralized chemicals (Orchard et al., 2000).
Phytotoxic effects were determined based on mass and size decrease and compared to chemical concentrations to determine toxic doses. Mass and size phytotoxic effects of chemicals were measured by relative growth rates (RGR) and Leaf Area Ratio (LAR). The toxic doses were usually reported as EC50 and LD50.
Phytotoxic effects to plants were measured indirectly in relation to photosynthesis. A decline in a plants ability to photosynthesize correlated with chlorophyll pigment reduction, which in turn was an indicator of more phytotoxic damage downstream. Several studies used non-destructive chlorophyll fluorometers to measure photosynthesis rates as they related to PAH toxicity (Slaski et al., 2002; Kummerova et al., 2007; Kapustka et al., 2004).
Chemical concentrations in solution and plant tissue required a variety of instruments ranging from Nuclear Magnetic Resonance (NMR), Inductively Couple Plasma (ICP), Atomic Absorption Spectrophotometry (AAS), Gas Chromatography (GC), and High Pressure Liquid Chromatography (HPLC).
Mechanisms of Action:
The materials and methods were often used to predict the mechanism of action (MOA) driving chemical uptake or phytotoxicity. The effect of PAHs on plants was frequently studied and the MOA driving toxicity was explained on various levels. For example, some studies simply stated that PAHs affected the chlorophyll, others stated that the damage was done in the thylakoid membrane where electron transport reactions occur (Marwood et al., 2003), and others reported MOA on the protein level (i.e. CYP-450 oxidation) (Kummerova et al., 2007). Reports show that PAH occurs naturally and anthropogenically as a parent compound (Marwood et al., 2003), but becomes more toxic after the metabolites are formed (El-Alawi et al., 2002) which can be an order of magnitude more toxic (Kummerova et al., 2007). PAH could be absorbed in solution or from the air, the principal toxic by-product is “UV-mediated photo-modification and subsequent disruption of photosynthesis [on aquatic plants]” (Kapustka et al., 2004). “Plants [uptake], translocate, transform, and accumulate PAHs,” and are responsible for eliminated up to 45% of the PAHs from the environment (Kummerova et al., 2007).
The MOA induced by Cu and Cd toxicity created free radicals that led to peroxidation of membrane lipids, which then led to loss of membrane integrity. “Plasma membrane permeability [could] result in leakage of potassium ions and other solutes and, finally, cause cell death” (Wang et al., 2004).
Uranium can enter inside of cells via mechanisms like: i) biomethylisation, which uptakes Ur via Methylobacterium spp. that symbiotically feed off of toxic plant by-products like CH4 (Taiz et al.); ii) assimilation, and iii) compartamentalization with specific enzymes (Makandawire et al., 2004, 2005).
Certain enzymes are only activated due to phytotoxicity and not physical cell damage. These enzymes include peroxidases (i.e. duckweed SpEx), superoxide dismutase, guaiacol peroxidase, and ascorbate peroxidase (Jansen et al., 2004; Wang et al., 2004). These enzymes were activated—toxicity indicator—after exposure with 2,4,6-trichlorophenol (TCP) and Copper. Chlorophenols were also enzymatically turned into glucoconjugates and incorporated into vacuoles and cell walls (Day et al., 2004).
Several MOAs were identified in a study observing the effect of duckweed exposure to pharmaceuticals. The studies quantified toxicity with plant necrosis, chlorophyll pigment, and carotenoid. The studies showed that fluoroquinolone-, sulfonamide-, and tetracycline-type compounds had the most toxic effect on duckweed. Fluoroquinolone toxicity caused bleaching of new fronds due to “inhibiting the activity of DNA gyrases.” The MOA of sulfamethoxazole is that they’re “folate antoagonists [which block] the conversion of p-aminobenzoid acid to the coenzyme dihydrofolic acid.” And the chlortetracycline inhibited protein synthesis (Brain et al., 2004, 2006).
Chemicals Studied:
Duckweed is suitable for phytoremediation of polluted surface water. It has demonstrated an ability to hyperaccumulate N, P, Cu, Cd, and Zn. Additionally, it accumulates more toxic metals such as As, Ag, Al, Cr, Fe, Hg, Ni, Pb, Ur (Objegba, Wang et al., 2004; Mkandawire et al., 2005, Olguin et al., 2005).
Organic chemicals taken up by duckweed and other plants include: N-nitrosodimethylamine (NDMA), perchlorate, trichloroethylene (TCE), di-chlorophenol (4-Cl-2-FP), triphenyltin, naphthalene, creosote, 2,4,6-trichlorophenol (TCP), chlorobenzene, and phenanthrene, plus various pharmaceuticals which have been found in the environment.
Engineering Significance & Applications:
The plants in the studies for this review were used to measure chemical uptake and toxicity in the following applications: Treatment of wastewater, mine drainage water, flue gas water, and aquatic pollution in wetlands, rivers, and lakes. Referring back to the canary in the coal mine analogy, plants like duckweed have the potential to point out the hazards associated with certain chemicals (some of which might be harmful to human health), while also removing these chemicals from impaired waters.
References:
Alaerts, G. J., Mahbubar, M. R., Kelderman, P. (1996). Peformance analysis of a full-scale duckweed-covered sewage lagoon. Water Research. 30(4), 843-852.
Boniardi, N., R. Rota, et al. (1999). Effect of dissolved metals on the organic load removal efficiency of Lemna gibba. Water Research 33, 530-538.
Bottcher, T. and R. Schroll (2007). The fate of isoproturon in a freshwater microcosm with Lemna minor as a model organism. Chemosphere. 66(4), 684-689.
Boutonnet, J. C., P. Bingham, et al. (1999). Environmental risk assessment of trifluoroacetic acid. Human and Ecological Risk Assessment. 5(1), 59-124.
Brain, A. R., Johnson, D. J., Richards, S. M., Hanson, M. L., Sanderson, H., Lam, M. W., Young, C., Mabury, S. A., Sibley, P. K., Solomon, K. R. (2004). Microcosm evaluation of the effects of an eight pharmaceutical mixture to the aquatic macrophytes Lemna gibba and Myriophyllum sibiricum. Aquatic Toxicology. 70, 23-40.
Brain, R. A., Sanderson, H., Sibley, P. K., Solomon, K. R. (2006). Probabilistic ecological hazard assessment, Evaluating pharmaceutical effects on aquatic higher plants as an example.
Ecotoxicology and Environmental Safety. 64, 128-135.
Chaiprapat S., Cheng, J. J., Classen, J. J., Liehr, S. K. (2005). Role of internal nutrient storage in duckweed growth for swine wastewater treatment. Trnasaction of the ASAE. 48(6), 2247-2258.
Clatworthy, J. N., Harper, J. L. (1960). The comparative biology of closely related species living in the same area. Comparative Biology. 307-324.
Culley Jr., D. D., Rejmankova, E., Kvet, J., Frye, J. B. (1981) Production, chemical, quality, and use of duckweed (Lemnaceae) in aquaculture, waste management, and animal feeds. Journal of World Mariculture Society. 12(2), 27-49.
Day, J. A. and F. M. Saunders (2004). Glycosidation of chlorophenols by Lemna minor. Environmental Toxicology and Chemistry. 23(3), 613-620.
Devi, M., D. A. Thomas, et al. (1996). Accumulation and physiological and biochemical effects of cadmium in a simple aquatic food chain. Ecotoxicology and Environmental Safety. 33(1), 38-43.
Donahue, D. (2009) Personal Correspondence.
Edwards, P., Hassan, M. S., Chao, C. H., Pacharaprakiti, C. (1992). Cultivation of duckweeds in setage-loaded earthen ponds. Bioresource Technology. 40, 109-117.
El-Alawi, Y. S., X. D. Huang, et al. (2002). Quantitative structure-activity relationship for the photoinduced toxicity of polycyclic aromatic hydrocarbons to the luminescent bacteria Vibrio fischeri. Environmental Toxicology and Chemistry. 2110), 2225-2232.
Gomez-Hermosillo, C., Pardue J. H., et al. (2006). Wetland plant uptake of desorption-resistant organic compounds from sediments. Environmental Science & Technology 40, 3229-3236.
Jansen, M. A. K., L. M. Hill, et al. (2004). A novel stress-acclimation response in Spirodela punctata (Lemnaceae), 2,4,6-trichlorophenol triggers an increase in the level of an extracellular peroxidase, capable of the oxidative dechlorination of this xenobiotic pollutant. Plant Cell and Environment. 27(5), 603-613.
Kapustka, L. A. (2004). Establishing Eco-SSLs for PAHs, Lessons revealed from a review of literature on exposure and effects to terrestrial receptors. Human and Ecological Risk Assessment. 10(2), 185-205.
Kim, J., M. C. Drew, et al. (2004). Uptake and phytotoxicity of TNT in onion plant. Journal of Environmental Science and Health Part a-Toxic/Hazardous Substances & Environmental Engineering. 39(3), 803-819.
Kummerova, M., S. Zezulka, et al. (2007). Photoinduced toxicity of fluoranthene on primary processes of photosynthesis in lichens. Lichenologist. 39, 91-100.
Linton, S., Goulder, R. (1998). The duckweed Lemna minor compared with the alga Selenastrum capricornutum for bioassay of pond-water richness. Aquatic Botany. 60, 7-36.
Lone, M. I., He Z., Stoffella, P. J., Yang, X. (2008). Phytoremediation of heavy metal polluted soils and water: progresses and perspectives. University of Science B. 9, 210-220.
Marwood, C. A., K. T. J. Bestari, et al. (2003). Creosote toxicity to photosynthesis and plant growth in aquatic microcosms. Environmental Toxicology and Chemistry. 22(5), 1075-1085.
Mkandawire, M. and E. G. Dudel (2005). Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Science of the Total Environment. 336(1-3), 81-89.
Mkandawire, M., B. Tauert, et al. (2004). Capacity of Lemna gibba L. (Duckweed) for uranium and arsenic phytoremediation in mine tailing waters. International Journal of Phytoremediation. 6(4), 347-362.
Nzengung, V. A., H. Penning, et al. (2004). Mechanistic changes during phytoremediation of perchlorate under different root-zone conditions. International Journal of Phytoremediation. 6(1), 63-83.
Odjegba, V. J. and I. O. Fasid (2004). Accumulation of trace elements by Pistia stratiotes, implications for phytoremediation. Ecotoxicology. 13(7), 637-646.
Olguin, E. J., G. Sanchez-Galvan, et al. (2005). Surface adsorption, intracellular accumulation and compartmentalization of Pb(II) in batch-operated lagoons with Salvinia minima as affected by environmental conditions, EDTA and nutrients. Journal of Industrial Microbiology & Biotechnology. 32(11-12), 577-586.
Orchard, B. J., W. J. Doucette, et al. (2000). A novel laboratory system for determining fate of volatile organic compounds in planted systems. Environmental Toxicology and Chemistry. 19(4), 888-894.
Ornes, W. H., K. S. Sajwan, et al. (1991). Bioaccumulation of Selenium by Floating Aquatic Plants. Water Air and Soil Pollution. 57-8, 53-57.
Prasad, M. N. V. and H. M. D. Freitas (2003). Metal hyperaccumulation in plants - Biodiversity prospecting for phytoremediation technology. Electronic Journal of Biotechnology. 6(3), 285-321.
Reinhold, D. M. and E. M. Saunders (2006). Phytoremediation of fluorinated agrochemicals by duckweed. Transactions of the Asabe. 49(6), 2077-2083.
Saygideger, S. and M. Dogan (2004). Lead and cadmium accumulation and toxicity in the presence of EDTA in Lemna minor L. and Ceratophyllum demersum L. Bulletin of Environmental Contamination and Toxicology. 73(1), 182-189.
Slaski, J. J., D. J. Archambault, et al. (2002). Physiological tests to measure impacts of gaseous polycyclic aromatic hydrocarbons (PAHs) on cultivated plants. Communications in Soil Science and Plant Analysis. 33(15-18), 3227-3239.
Sundberg, S. E., S. M. Hassan, et al. (2006). Enrichment of elements in detritus from a constructed wetland and consequent toxicity to Hyalella azteca. Ecotoxicology and Environmental Safety. 64(3), 264-272.
Taiz, E., Zeiger, E. (1998). Plant Physiology. 2nd ed. 147-152; 173-193.
Tront, J. M. and F. M. Saunders (2006). Role of plant activity and contaminant speciation in aquatic plant assimilation of 2,4,5-trichlorophenol. Chemosphere. 64(3), 400-407.
Tront, J. M. and F. M. Saunders (2007). Sequestration of a fluorinated analog of 2,4-dichlorophenol and metabolic products by L-minor as evidenced by F-19 NMR. Environmental Pollution. 145(3), 708-714.
Vadas, T. M., X. Zhang, et al. (2007). Fate of DTPA, EDTA, and EDDS in hydroponic media and effects on plant mineral nutrition. Journal of Plant Nutrition. 30(7-9), 1229-1246.
Wang, H., X. Q. Shan, et al. (2004). Responses of antioxidative enzymes to accumulation of copper in a copper hyperaccumulator of Commoelina communis. Archives of Environmental Contamination and Toxicology. 47(2), 185-192.
Wang, Q., Y. Cui, et al. (2002). Phytoremediation of polluted waters potentials and prospects of wetland plants. Acta Biotechnologica. 22(1-2), 199-208.
Yifru, D. D. and V. A. Nzengung (2006). Uptake of N-nitrosodimethylamine (NDMA) from water by phreatophytes in the absence and presence of perchlorate as a co-contaminant. Environmental Science & Technology. 40(23), 7374-7380.


 Some aquatic species tolerate cold climates as well or better than duckweed but unlike duckweed they do not currently grow on Wellsville nor Logan wastewater lagoons in Cache Valley. Watercress (Nasturtium officinale), have the ability to grow during the harsh Cache Valley winters provided that they are near flowing water like springs [Michaelis, 1976; personal observation]. Watercress grows during the winter in a canal running adjacent and north of Canyon Road in Logan, Utah, near the Utah Water Research Laboratory. Duckweed favors calm water, unfortunately, this turns to ice and limits the duckweed growing season to approximately six months in Cache Valley.
Some aquatic species tolerate cold climates as well or better than duckweed but unlike duckweed they do not currently grow on Wellsville nor Logan wastewater lagoons in Cache Valley. Watercress (Nasturtium officinale), have the ability to grow during the harsh Cache Valley winters provided that they are near flowing water like springs [Michaelis, 1976; personal observation]. Watercress grows during the winter in a canal running adjacent and north of Canyon Road in Logan, Utah, near the Utah Water Research Laboratory. Duckweed favors calm water, unfortunately, this turns to ice and limits the duckweed growing season to approximately six months in Cache Valley.  Figure 2: Duckweed fronds 3-7 days after Fig. 1.
Figure 2: Duckweed fronds 3-7 days after Fig. 1. Figure 3: Duckweed fronds completely infected by fungi and being decomposed by micro-organisms.
Figure 3: Duckweed fronds completely infected by fungi and being decomposed by micro-organisms. Figure 6: Signs of algae infestation (progressively worse L to R).
Figure 6: Signs of algae infestation (progressively worse L to R). Figure 7 (top-bottom pairs): Logan City wastewater w/o and w/ chlorine;
Figure 7 (top-bottom pairs): Logan City wastewater w/o and w/ chlorine; Figure 8: Bioassays (APHA Std. Method 8211) by frond count. Logan w/ and w/o chlorine.
Figure 8: Bioassays (APHA Std. Method 8211) by frond count. Logan w/ and w/o chlorine. Figure 9: Bioassay method 8211 Hydrosol and Wellsville City ww solutions.
Figure 9: Bioassay method 8211 Hydrosol and Wellsville City ww solutions.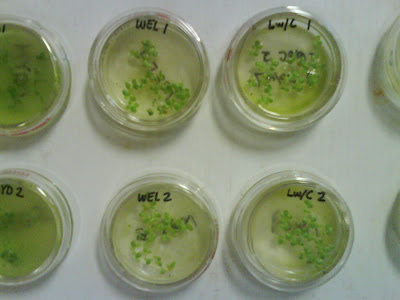 Figure 10: Bioassay method 8211; 3-5 days (typ. 96 hrs.); Wellville City and Logan City w/Cl ww.
Figure 10: Bioassay method 8211; 3-5 days (typ. 96 hrs.); Wellville City and Logan City w/Cl ww. Figure 11: Duckweed plants following bioassay;
Figure 11: Duckweed plants following bioassay; Figure 12: More duckweed plants following bioassay;
Figure 12: More duckweed plants following bioassay; Figure 13: Bioassay results comparing effect of nutrient solutions and chlorination on duckweed growth. It appears that nutrient solution has a larger effect on duckweed growth than whether or not it has been chlorinated (see below).
Figure 13: Bioassay results comparing effect of nutrient solutions and chlorination on duckweed growth. It appears that nutrient solution has a larger effect on duckweed growth than whether or not it has been chlorinated (see below).

 Figure: Temperature Trend two weeks preceding video on 15 Jan 2011--Cache Valley Winter
Figure: Temperature Trend two weeks preceding video on 15 Jan 2011--Cache Valley Winter
